Abstract
Background: Patients who fail hypomethylating agent (HMA) therapy for the treatment of myelodysplastic syndrome (MDS) have poor outcomes and lack of treatment options. Immune checkpoint inhibitors (ICPIs) including the programmed cell-death protein-1 (PD-1) inhibitor nivolumab, and cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) inhibitor ipilimumab, have demonstrated activity in the treatment of MDS, including those who have failed prior HMA therapy. Toxicities due to ICPIs are a result of over activation of the immune system, requiring management with corticosteroids or additional immunosuppressants. The toxicity profile of nivolumab and ipilimumab alone and in combination has not been previously reported in patients with MDS.
Methods: Adverse events (ADEs) of adult patients treated with the ICPIs nivolumab and/or ipilimumab as monotherapy or in combination with azacitidine, as part of clinical trial NCT02530463, were retrospectively analyzed. ADE management with steroids and other immunosuppressant therapy was reviewed. Adverse events due to azacitidine were excluded (i.e. fatigue, nausea). Steroid treatment administered outside of MD Anderson Cancer Center was not captured. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
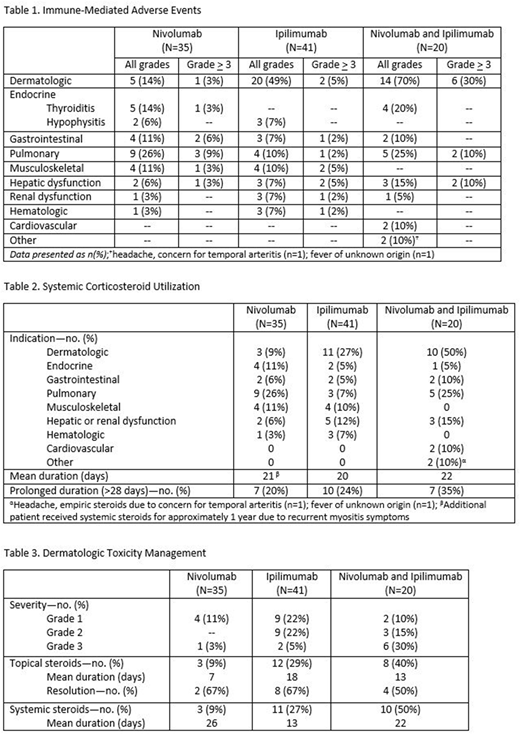
Results: Ninety-six patients treated with nivolumab (n=35), ipilimumab (n=41), or the combination of both nivolumab and ipilimumab (n=20) were evaluated. Observed immune toxicities varied between nivolumab and ipilimumab treatment arms (Table 1). Patients treated with ipilimumab experienced higher rates of dermatologic toxicity (n=20, 49%) compared to nivolumab (n=5, 14%), however pneumonitis was more frequently observed with nivolumab compared to ipilimumab (Table 1). The combination of nivolumab and ipilimumab led to higher rates of liver function test elevations (n=3, 15%) compared to either nivolumab (n=2, 6%) or ipilimumab (n=3, 7%) alone. Cardiovascular toxicity including myopericarditis (n=1) and presumed myocarditis (n=1) was only observed in the combination group. Hematologic toxicities occurred with both nivolumab (n=1, 3%) and ipilimumab (n=3, 7%) alone, but not with the combination. The rate of hypophysitis was 6% and 7% in the nivolumab and ipilimumab alone arms, respectively. Thyroiditis was only noted in the nivolumab alone (n=5, 14%) and combination arms (n=4, 20%). Systemic corticosteroids were routinely used to manage immune-mediated ADEs (Table 2). The average duration of systemic steroid treatment was similar between groups, although those in the combination arm more frequently required prolonged (>28 days) steroid courses (n=7, 35%) compared to ipilimumab (n=10, 24%) or nivolumab (n=7, 20%) alone. Topical steroids were utilized for grade 1-2 dermatologic toxicities. A greater proportion of patients with dermatologic toxicities resolved with topical steroids alone in the nivolumab (n=2, 67%) and ipilimumab (n=8, 67%) arms compared to the combination arm (n=4, 50%) (Table 3). Due to the higher rate of grade 3 dermatologic events in the combination arm (n=6, 30%) compared to either nivolumab (n=1, 3%) or ipilimumab (n=2, 5%) alone, more patients received systemic steroids to manage this toxicity. Four patients required additional immunosuppressive therapy, all of whom had received ipilimumab alone (n=2) or in combination (n=2). Patients in the ipilimumab group received rituximab for autoimmune hemolytic anemia (n=1), and tocilizumab with dexamethasone and etoposide for presumed hemophagocytic lymphohistiocytosis (n=1). Patients in the combination arm received cyclosporine to manage multiple immune-mediated ADEs including steroid-refractory dermatologic toxicity and colitis (n=1); as well as infliximab, cyclophosphamide, IVIG, rituximab, and mycophenolate mofetil for steroid-refractory pneumonitis (n=1).
Conclusions: The toxicities observed in patients with MDS treated with nivolumab and ipilimumab are consistent with those previously reported with these agents in other patient populations. Therapy combining nivolumab and ipilimumab led to higher rates of dermatologic, hepatic, and cardiovascular toxicities. Patients treated in the combination arm more frequently required prolonged courses of systemic corticosteroids (>28 days), and only those who received ipilimumab required additional immunosuppressant therapy.
Daver:Karyopharm: Consultancy; BMS: Research Funding; Sunesis: Consultancy; Sunesis: Research Funding; Novartis: Research Funding; ARIAD: Research Funding; Novartis: Consultancy; ImmunoGen: Consultancy; Otsuka: Consultancy; Incyte: Research Funding; Pfizer: Research Funding; Karyopharm: Research Funding; Kiromic: Research Funding; Incyte: Consultancy; Daiichi-Sankyo: Research Funding; Pfizer: Consultancy; Alexion: Consultancy. DiNardo:Abbvie: Honoraria; Agios: Consultancy; Medimmune: Honoraria; Karyopharm: Honoraria; Bayer: Honoraria; Celgene: Honoraria. Ravandi:Xencor: Research Funding; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding; Sunesis: Honoraria; Seattle Genetics: Research Funding; Abbvie: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Xencor: Research Funding; Jazz: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Orsenix: Honoraria; Jazz: Honoraria; Abbvie: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Macrogenix: Honoraria, Research Funding; Sunesis: Honoraria; Bristol-Myers Squibb: Research Funding; Orsenix: Honoraria. Bose:Pfizer, Inc.: Research Funding; Astellas Pharmaceuticals: Research Funding; Constellation Pharmaceuticals: Research Funding; Blueprint Medicines Corporation: Research Funding; CTI BioPharma: Research Funding; Celgene Corporation: Honoraria, Research Funding; Incyte Corporation: Honoraria, Research Funding. Pemmaraju:SagerStrong Foundation: Research Funding; Affymetrix: Research Funding; plexxikon: Research Funding; daiichi sankyo: Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; abbvie: Research Funding; cellectis: Research Funding; stemline: Consultancy, Honoraria, Research Funding; novartis: Research Funding. Cortes:Novartis: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Arog: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Kadia:Jazz: Consultancy, Research Funding; BMS: Research Funding; Celgene: Research Funding; Celgene: Research Funding; Pfizer: Consultancy, Research Funding; Abbvie: Consultancy; Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Abbvie: Consultancy; Amgen: Consultancy, Research Funding; Novartis: Consultancy; BMS: Research Funding; Takeda: Consultancy; Novartis: Consultancy; Jazz: Consultancy, Research Funding; Takeda: Consultancy. Konopleva:Stemline Therapeutics: Research Funding. Jain:Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; BMS: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Adaptive Biotechnologioes: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Adaptive Biotechnologioes: Research Funding; Pharmacyclics: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Research Funding; Cellectis: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; Infinity: Research Funding; Servier: Research Funding; ADC Therapeutics: Research Funding; Genentech: Research Funding; Astra Zeneca: Research Funding; Celgene: Research Funding; BMS: Research Funding; Pfizer: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Infinity: Research Funding; Genentech: Research Funding; Abbvie: Research Funding; Pharmacyclics: Research Funding; Cellectis: Research Funding; Verastem: Research Funding. Andreeff:Aptose: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Patents & Royalties: MDM2 inhibitor activity patent, Research Funding; Reata: Equity Ownership; Oncoceutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Research Funding; Oncolyze: Equity Ownership; SentiBio: Equity Ownership; Celgene: Consultancy; Astra Zeneca: Research Funding; Jazz Pharma: Consultancy; United Therapeutics: Patents & Royalties: GD2 inhibition in breast cancer ; Eutropics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal